While the risk to the public remains low, the highly pathogenic avian influenza (HPAI) A(H5N1) is on the radar of those in sectors like livestock breeding, animal sciences and food production.
Published May 28, 2024
By Syra Madad, DHSc; Jason Kindrachuk, PhD; and Rick A. Bright, PhD

Recent observations on highly pathogenic avian influenza (HPAI) A(H5N1) have highlighted the virus’s transmission among dairy cattle in the United States. Key findings include ongoing detection and transmission of H5N1 among cattle, a second human case of H5N1 infection in a farmworker; mixed virus receptor distribution in mammary gland tissue of cattle, genetic evolution of H5N1 with onward transmission, evaluation of pasteurization effectiveness for virus inactivation, and a clinical description of HPAI H5N1 influenza A virus infection in a U.S. dairy farm worker.
Genomic and Epidemiologic Insights
In May 2024, investigators at the U.S. Department of Agriculture (USDA) reported genomic and epidemiologic data showing HPAI A(H5N1) spillover to, and transmission among, cattle. While prior data on Influenza A virus in cattle is scarce, the current geographic expansion of HPAI H5N1 among herds across multiple U.S. states demonstrates clade 2.3.4.4b’s affinity for cattle.
Reduced food intake, milk production, and shifting milk quality was first noted in January 2024, followed by detection of influenza A virus, specifically H5N1 clade 2.3.4.4b genotype B3.13, by the National Animal Health Laboratory Network and National Veterinary Services Laboratories. Subsequent analysis suggested movement of genotype B3.13 between dairy cattle farms and domestic poultry.
The study’s authors suggested a single spillover event from wild birds with limited cattle-to-cattle transmission around December 2023. Additional spillovers were identified from infected cattle to poultry and other nearby mammals, with the virus potentially shedding from infected cattle for 14-21 days. Genome sequencing indicated ongoing evolution, possibly linked to mammalian adaptation.
Viral Receptor Distribution
Sialic acid receptors utilized by influenza A viruses for cellular attachment, are found in multiple cattle tissues, including the respiratory tract, mammary glands, and brain. Though all type of sialic acid receptors could be found in each of these areas, the types and concentration of sialic acid receptors varied by tissue; those used by human and duck viruses were more prominent in the mammary gland and to a lesser degree in the respiratory tract, while those used by chicken viruses were more prominent in the respiratory tract and to a lesser degree in the mammary glands.
These findings provide insights into HPAI A(H5N1)’s tissue tropism in cattle and its transmission patterns. The presence of multiple types of species-specific receptors for influenza A viruses located throughout the dairy cattle also permits hypotheses on potential for them to serve as a mixing vessel for accelerated reassortment of influenza viruses, increasing a potential for the evolution of an influenza A virus with human pandemic potential.
Pasteurization and Food Safety
On May 1, 2024, the U.S. Food and Drug Administration confirmed that pasteurization inactivates H5N1 virus in a variety of milk products. No infectious H5N1 virus was found in nearly 300 retail dairy samples that were positive for viral nucleic acid by quantitative PCR. Additionally, neither viral nucleic acid nor infectious virus was found in retail powdered infant formula and powdered milk. This supports pasteurization’s effectiveness in inactivating concentrations of H5N1 virus found in the milk supply among samples collected in April. Advisories against consuming raw/unpasteurized milk or milk products remain in place.
Clinical Case in a Dairy Farm Worker
A recent study reported on the first reported human case of H5N1 infection in a U.S. dairy farm worker who experienced ocular discomfort without respiratory symptoms or fever. The worker had close contact with symptomatic dairy cows from farms with confirmed H5N1 infections. Personal protective equipment included gloves but no ocular protection. Swab specimens from the conjunctiva and nasopharynx confirmed H5N1 through RT-PCR and viral genome sequencing. Home isolation and oral oseltamivir were recommended, leading to resolution of conjunctivitis.
No secondary infections were reported among household contacts. Importantly, viral sequences showed no mutations suggesting changes in receptor binding or antiviral susceptibility. However, a mutation in the internal PB2 gene showed a change that is more commonly associated with human adaptation and warrants close monitoring.
Implications and Recommendations
These reports underscore the need for comprehensive HPAI A(H5N1) surveillance in agricultural settings. While cattle infections have been reported by the USDA to be generally transient with mild symptoms, the potential impact on milk production and food security is significant. The risk of ongoing viral evolution and broad transmission among cattle could lead to further mammalian adaptation. Although human infections from cattle seem to be rare at this time, the burden of infection necessitates detailed assessments of human spillovers, especially in areas with current or prior outbreaks. This includes serology to establish spillover rates to humans and monitor for changes in spillover frequency.
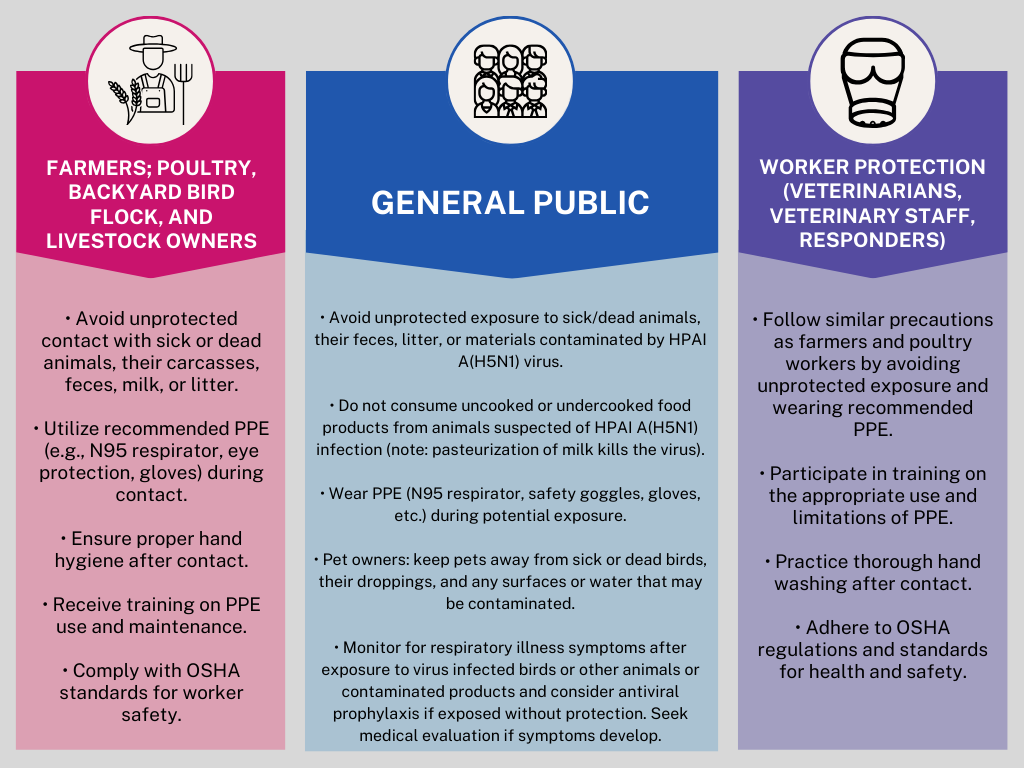
While the general public’s risk remains low, those at higher risk include individuals with routine or frequent contact with potentially infected birds, livestock, other animals or contaminated animal products and environments (e.g., farmers, livestock workers, animal handlers, employees of milk and meat processing facilities, milk or carcass transport drivers, and veterinarians).
Human infections with H5N1 can occur when the virus enters the eyes, nose, or mouth, or is inhaled. This can happen through airborne droplets, small aerosol particles, or dust that settles on mucous membranes. Infection can also occur if a person touches a contaminated surface and then touches their mouth, eyes, or nose. Exposed individuals should monitor for symptoms within 10 days, including fever (100°F [37.8°C] or higher), chills, cough, sore throat, difficulty breathing/shortness of breath, eye tearing, redness, or irritation, headaches, runny or stuffy nose, muscle aches, and diarrhea.

About the Authors
Syra Madad, DHSc, MSc, MCP, CHEP is an internationally renowned epidemiologist in special pathogens preparedness and response, biosecurity advisor and science communicator. She serves as the Chief Biopreparedness Officer at NYC Health + Hospitals, the U.S.’s largest municipal healthcare delivery system. Dr. Madad is a fellow at Harvard University’s Belfer Center for Science and International Affairs where she leads the Women in STEM and Diversity in STEM series; she’s Core Faculty at the National Emerging Special Pathogens Training and Education Center (NETEC), and affiliate faculty at Boston University’s Center on Emerging Infectious Diseases.
Her work focuses on the prevention, preparedness, response, and recovery from infectious disease outbreaks with an emphasis on healthcare and public health biopreparedness. She is known for her innovative strategies, which integrate emergency management principles with epidemiological methods, contributing significantly to the development of robust healthcare systems that can respond to emerging disease threats. You can follow her on X (twitter) and Instagram: @syramadad
Read more from Dr. Madad on the Academy blog: The COVID-19 Pandemic at Year Four: The Imperative for Global Health Solidarity; Crossing Species: The Rising Threat of H5N1 Bird Flu in the U.S.
Jason Kindrachuk, PhD is an Associate Professor, Canada Research Chair, Department of Medical Microbiology & Infectious Diseases, University of Manitoba, Winnipeg, MB, Canada.
Rick A. Bright, PhD is CEO, Bright Global Health and Former Deputy Assistant Secretary for Preparedness and Response, U.S. Department of Health and Human Services.